TP9
Generation and neurobiological characterization of human cerebral organoids modelling Rasopathies
One widely used option to model neurodevelopmental disorders is to generate and characterise mice harboring the underlying genetic mutations (1). Targeted manipulation of the appropriate genes followed by a broad neurobiological analysis of the corresponding mutants does reveal phenotypes that might further contribute to a better understanding of pathomechanisms and pave the way for the development of translational therapies (2). Previously, we have generated and neurobiologically characterized mouse models for neurodevelopmental disorders involving SHANK mutations, i.e. the Phelan-McDermid Syndrome, which mainly presents with intellectual disability (ID) and autistic-like features – both present also in Rasopathies such as cardiofaciocutaneous (CFC) or Noonan syndrome (1-5). However, evidence from several fields of neuroscience research has emerged over the last decade that mice might not always serve to close the translational gap between animal models and human patients (6,7). That’s why in this subproject we will generate human patient induced pluripotent stem cell (iPSC)-derived three-dimensional structures resembling the developing human brain called cerebral organoids (8,9) carrying mutations in Rasopathy genes. Our laboratory is already closely collaborating with the laboratory of Prof. Gaia Novarino at the Institute of Science and Technology Austria (IST Austria, Klosterneuburg) who has established this method together with its inventors (Knoblich group, IMBA Vienna). These organoids should finally serve to model neuronal Rasopathy phenotypes for the first time as closely to the affected individuals as possible. We will then apply the state-of-the-art neuroanatomical and neuromolecular methods to characterise these organoids in detail in order to better understand the pathoneurobiology of Rasopathies in a tissue-like human brain model system.
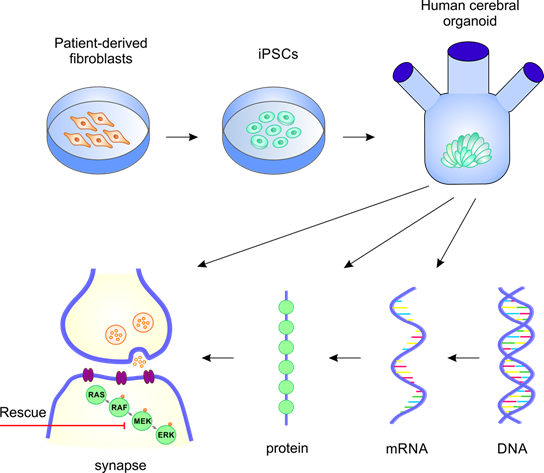
Legend: Using patient-derived material, iPSCs will be generated and further used to produce human cerebral organoids. These organoids, carrying mutations in Rasopathy genes, will be characterized using neuromolecular and neuroanatomical methods. The cellular and molecular processes, likely affected by these mutations, will be studied at transcriptional, translational and functional levels in the frame of our project.
Major goals:
- Generation of human patient-derived cerebral organoids carrying mutations in Rasopathy genes.
- Neuroanatomical and neuromolecular characterization of human cerebral organoids modelling Rasopathies.
- Restoration of phenotypes using genome editing technology.
Publications:
- Schmeisser MJ Ey E, Wegener S, Bockmann J, Stempel AV, Kuebler A, Janssen AL, Udvardi PT, Shiban E, Spilker C, Balschun D, Skryabin BV, Tom Dieck S, Smalla KH, Montag D, Leblond CS, Faure P, Torquet N, Le Sourd AM, Toro R, Grabrucker AM, Shoichet SA, Schmitz D, Kreutz MR, Bourgeron T, Gundelfinger ED, Boeckers TM: Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature. 2012;486(7402): 256-60. PMID: 22699619
- Schroeder, JC, Reim D, Boeckers TM, Schmeisser MJ: Genetic Animal Models for Autism Spectrum Disorder. Curr Top Behav Neurosci. 2017;30:311-24. PMID: 26602248
- Vicidomini C, Ponzoni L, Lim D, Schmeisser MJ, Reim D, Morello N, Orellana D, Tozzi A, Durante V, Scalmani P, Mantegazza M, Genazzani AA, Giustetto M, Sala M, Calabresi P, Boeckers TM, Sala C, Verpelli C: Pharmacological enhancement of mGlu5 receptors rescues behavioral deficits in SHANK3 knock-out mice. Mol Psychiatry. 2017;22(5):689-702. PMID: 27021819
- Reim D, Distler U, Halbedl S, Verpelli C, Sala C, Bockmann J, Tenzer S, Boeckers TM, Schmeisser MJ: Proteomic analysis of postsynaptic denstiy fractions from Shank3 mutant mice reveals brain region specific changes relevant to autism spectrum disorder. Front Mol Neurosci. 2017;10:26. PMID: 28261056
- Garg S, Brooks A, Burns A, Burkitt-Wright E, Kerr B, Huson S, Emsley R, Green J: Autism spectrum disorder and other neurobehavioural comorbidities in rare disorders of the Ras/MAPK pathway. Dev Med Child Neurol. 2017, 59(5):544-549. PMID: 28160302
- Geerts H: Of mice and men: bridging the translational disconnect in CNS drug discovery. CNS Drugs. 2009, 23(11):915-926. PMID: 19845413
- Windisch M: We can treat Alzheimer’s disease successfully in mice but not in men: failure in translation? A perspective.Neurodegener Dis. 2014, 13(2-3):147-150. PMID: 24401335
- Lancaster MA, Renner M, Martin CC, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA: Cerebral organoids model human brain development and microcephaly. Nature. 2013, 501(7467):373-379. PMID: 23995685
- Lancaster MA, Knoblich JA: Generation of cerebral organoids from human pluripotent stem cells. Nat Protoc. 2014, 9(10):2329- 2340. PMID: 25188634
- Mariani J, Coppola G, Zhang P, Abyzov A, Provini L, Tomasini L, Amenduni M, Szekely A, Palejev D, Wilson M, Gerstein M, Grigorenko EL, Chawarska K, Pelphrey KA, Howe JR, Vaccarino FM: FOXG1-Dependent Dysregulation of GABA/Glutamate Neuron Differentiation in Autism Spectrum Disorders. Cell. 2015, 162(2):375-390. PMID: 26186191
- Heise C, Schroeder JC, Schoen M, Halbedl S, Reim D, Woelfle S, Kreutz MR, Schmeisser MJ, Boeckers TM. Selective localization of Shanks to VGLUT1-positive excitatory synapses in the mouse hippocampus. Front Cell Neurosci 2016;10:106. PMID: 27199660
- Schroeder JC, Deliu E, Novarino G, Schmeisser MJ. Genetic and Pharmacological Reversibility of Phenotypes in Mouse Models of Autism Spectrum Disorder. Adv Anat Embryol Cell Biol. 2017, 224:189-211. PMID: 28551757







