TP5 - FP1
Molecular pathogenesis of RASopathy-associated hypertrophic cardiomyopathy and identification of new treatment strategies
The primary goal of this subproject is to understand the molecular cause of hypertrophic cardiomyopathy by carefully examining various cellular processes controlled by the SHP2 and RAF1 signal transduction. To this end, we use induced pluripotent stem (iPS) cells originated from primary fibroblast of individuals with Noonan Syndrome and Leopard Syndrome, obtained from TP1. These cells will be used both to investigate the basic mechanisms of pluripotency and to differentiate them to contractile cardiac muscle cells in two different ways, two-dimensional monolayer culture and three-dimensional cardiac bodies generated from embryoid bodies. A third way is iPS cell differentiated in the form of organoid `bioartificial cardiac tissue´ (BTC), which we obtain from TP1. The latter will be used in this collaborative study to analyze comprehensively signal transduction pathways downstream of SHP2 and RAF1. In addition to signal transduction profiling, we will conduct quantitative SILAC phosphoproteomics of myocardial cells to decipher regulatory networks disturbed by abnormal SHP2 and RAF1 activities. These investigations together with in situ protein-protein interactions by a detergent-free cell fractionation protocol and proximity ligation assay along with cDNA microarray and quantitative RT-PCR will ultimately disclose further molecular details of dysregulated signaling pathways. In this context, control cell lines will additionally be generated utilizing gene knockdown and editing techniques. We are convinced that deciphering new functional control mechanisms will identify optimal targets for drug screening in collaboration with `the Lead Discovery GmbH´ in Dortmund, our partners in the field of pharmaceutical drug discovery and research.
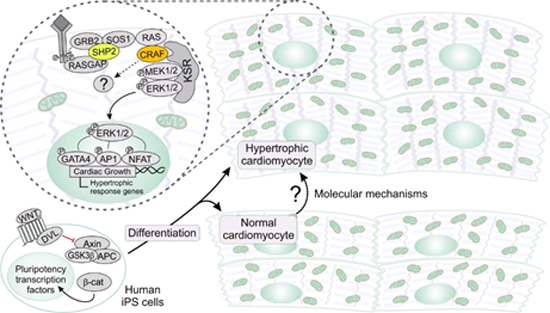
Human iPS cell models of RASopathy-associated HCM. Human iPS cell differentiation towards contractile cardiac muscle cells, which is achieved among others via modulation of the WNT pathway. This provide us a valuable tool to investigate the molecular mechanism of HCM in RASopathy patients.
Major goals:
- Exploring the molecular mechanisms of pluripotency and stem cell maintenance
- Generation and biochemical and physiological characterization of both contractile cardiac muscle cells originated from RASopathy-derived iPS cells and adult cardiomyocyte cell line HL-1
- Analyzing the protein interaction networks of SHP2 and RAF1 under different conditions in order to identify new biomarkers and/or molecular targets for HCM treatment
Publications:
Altmüller F, Lissewski C, Bertola D, Flex E, Stark Z, Spranger S, Baynam G, Buscarilli de Moraes M, Dyack S, Gillis J, Yntema HG, Pantaleoni F, van Loon RL, MacKay S, Mina K, Schanze I, Tan TY, Walsh M, White SM, Niewisch MR, García-Miñaúr S, Plaza D, Ahmadian MR, Cavé H, Tartaglia M, Zenker M. Genotype and phenotype spectrum of NRAS germline mutations. Eur J Hum Genet. 2017 Jun;25(7):823-831.
PMID: 28594414
Altmüller F, Pothula S, Annamneedi A, Nakhaei-Rad S, Montenegro-Venegas C, Pina-Fernández E, Marini C, Santos M, Schanze D, Montag D, Ahmadian MR, Stork O, Zenker M, Fejtova A. Aberrant neuronal activity-induced signaling and gene expression in a mouse model of RASopathy. PLoS Genet. 2017 Mar 27;13(3):e1006684.
PMID: 28346493
Boppudi S, Bögershausen N, Hove HB, Percin EF, Aslan D, Dvorsky R, Kayhan G, Li Y, Cursiefen C, Tantcheva-Poor I, Toft PB, Bartsch O, Lissewski C, Wieland I, Jakubiczka S, Wollnik B, Ahmadian MR, Heindl LM, Zenker M (2016) Specific mosaic KRAS mutations affecting codon 146 cause oculoectodermal syndrome and encephalocraniocutaneous lipomatosis. Clin Genet. 2016 Oct;90(4):334-42. PMID: 26970110
Nakhaeizadeh H, Amin E, Nakhaei-Rad S, Dvorsky R, Ahmadian MR (2016) The RAS-Effector Interface: Isoform-Specific Differences in the Effector Binding Regions. PLoS One. 2016 Dec 9;11(12):e0167145. PMID: 27936046








