TP1
Mutation spectrum, genotype phenotype correlations, natural history and clinical management of RASopathies
This subproject is focused on clinical genetic and translational aspects of RASopathies. Research activities are directed towards clinical classification, genotype phenotype correlations, and improvement of patient care for RASopathies. Directed by Martin Zenker, a pediatrician and clinical genetic scientist with long-standing expertise in RASopathies, this subproject aims at the further development of a comprehensive database for gene variations and standardized phenotype data, the exploration of correlations between specific genetic changes and disease course and prognosis, as well as the further development of specialized structures to improve patient care. The subproject also provides an important platform for translational approaches through its direct connection to patients and support groups.
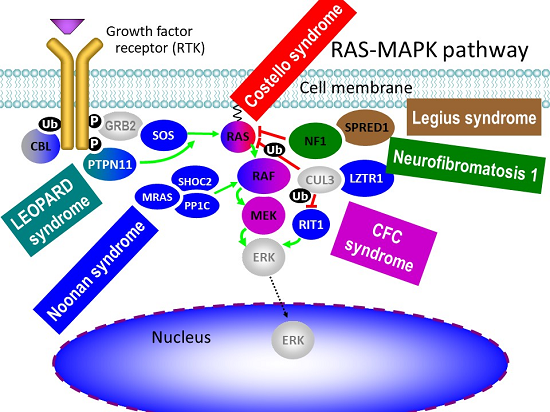
Legend: The RAS-MAPK pathway and associated human diseases caused by gain-of-fucntion germline mutations in its respective components.
The existing online mutation database (www.nseuronet.com), which was developed in 2010 as a mutation and genotype-phenotype database for RASopathies in the context of a previous ERA-Net for Research Programmes on Rare Diseases, will be continuously updated and equipped with novel software tools. A national patient registry will be developed and an interdisciplinary RASopathy clinics at the University Hospital Magdeburg will be further expanded and integrated into a national reference center for RASopathies under the umbrella of the “Mitteldeutsches Kompetenznetz für Seltene Erkrankungen” (MKSE: www.mkse.ovgu.de/Fachzentren).
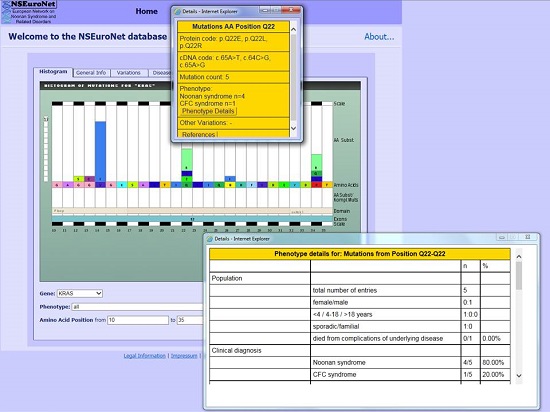
Legend: The NSEuroNet database is an updated online source for mutations in RASopathy genes and associated phenotypic features.
We are closely collaborating with family support groups:
Noonan-Kinder e.V.
CFC Angels e.V.
Major goals:
- Further development of the NSEuroNet database (www.nseuronet.com) as a comprehensive resource for the classification of variations in RASopathy genes
- Collection of standardized clinical and genetic data in order to explore genotype-phenotype correlations and natural history in large cohorts with defined subtypes of RASopathies
- Identification and characterization of novel genes and genetic mechanisms for RASopathies
- Establishment of a national patient registry
- Generation and further development of information materials on RASopathies for affected individuals and their families
- Collection of biomaterials from affected individuals for research goals fo the consortium
- Operation of genomic and transcriptomic analyses in collaboration with consortium partners
Publications:
- Capri Y, Flex E, Krumbach OHF, Carpentieri G, Cecchetti S, Lißewski C, Rezaei Adariani S, Schanze D, Brinkmann J, Piard J, Pantaleoni F, Lepri FR, Goh ES, Chong K, Stieglitz E, Meyer J, Kuechler A, Bramswig NC, Sacharow S, Strullu M, Vial Y, Vignal C, Kensah G, Cuturilo G, Kazemein Jasemi NS, Dvorsky R, Monaghan KG, Vincent LM, Cavé H, Verloes A, Ahmadian MR, Tartaglia M, Zenker M. Activating Mutations of RRAS2 Are a Rare Cause of Noonan Syndrome. Am J Hum Genet 2019; 104:1223-1232. PMID: 31130282
- Motta M, Sagi-Dain L, Krumbach OHF, Hahn A, Peleg A, German A, Lissewski C, Coppola S, Pantaleoni F, Kocherscheid L, Altmüller F, Schanze D, Logeswaran T, Chahrokh-Zadeh S, Munzig A, Nakhaei-Rad S, Cavé H, Ahmadian MR, Tartaglia M, Zenker M. Activating MRAS mutations cause Noonan syndrome associated with hypertrophic cardiomyopathy. Hum Mol Genet 2019 May 21. [Epub ahead of print] PMID: 31108500
- Motta M, Fidan M, Bellacchio E, Pantaleoni F, Schneider-Heieck K, Coppola S, Borck G, Salviati L, Zenker M, Cirstea IC, Tartaglia M. Dominant Noonan syndrome-causing LZTR1 mutations specifically affect the kelch domain substrate-recognition surface and enhance RAS-MAPK signaling. Hum Mol Genet 2018 Nov 27. PMID: 30481304
- Andelfinger G, Marquis C, Raboisson MJ, Théoret Y, Waldmüller S, Wiegand G, Gelb BD, Zenker M, Delrue MA, Hofbeck M. Hypertrophic Cardiomyopathy in Noonan Syndrome Treated by MEK-Inhibition. J Am Coll Cardiol 2019; 73:2237-2239. PMID: 31047013
- Haghighi F, Dahlmann J, Nakhaei-Rad S, Lang A, Kutschka I, Zenker M, Kensah G, Piekorz RP, Ahmadian MR. bFGF-mediated pluripotency maintenance in human induced pluripotent stem cells is associated with NRAS-MAPK signaling. Cell Commun Signal 2018; 16:96. PMID: 30518391
- Motta M, Fidan M, Bellacchio E, Pantaleoni F, Schneider-Heieck K, Coppola S, Borck G, Salviati L, Zenker M, Cirstea IC, Tartaglia M. Dominant Noonan syndrome-causing LZTR1 mutations specifically affect the Kelch domain substrate-recognition surface and enhance RAS-MAPK signaling. Hum Mol Genet 2019; 28:1007-1022. PMID: 30481304
- Schanze I, Bunt J, Lim JWC, Schanze D, Dean RJ, Alders M, Blanchet P, Attié-Bitach T, Berland S, Boogert S, Boppudi S, Bridges CJ, Cho MT, Dobyns WB, Donnai D, Douglas J, Earl DL, Edwards TJ, Faivre L, Fregeau B, Genevieve D, Gérard M, Gatinois V, Holder-Espinasse M, Huth SF, Izumi K, Kerr B, Lacaze E, Lakeman P, Mahida S, Mirzaa GM, Morgan SM, Nowak C, Peeters H, Petit F, Pilz DT, Puechberty J, Reinstein E, Rivière JB, Santani AB, Schneider A, Sherr EH, Smith-Hicks C, Wieland I, Zackai E, Zhao X, Gronostajski RM, Zenker M, Richards LJ. NFIB Haploinsufficiency Is Associated with Intellectual Disability and Macrocephaly. Am J Hum Genet 2018; 103:752-768. PMID: 30388402
- Grant AR, Cushman BJ, Cavé H, Dillon MW, Gelb BD, Gripp KW, Lee JA, Mason-Suares H, Rauen KA, Tartaglia M, Vincent LM, Zenker M. Assessing the gene-disease association of 19 genes with the RASopathies using the ClinGen gene curation framework. Hum Mutat. 2018; 39:1485-1493. PMID: 30311384
- Meyer Zum Büschenfelde U, Brandenstein LI, von Elsner L, Flato K, Holling T, Zenker M, Rosenberger G, Kutsche K. RIT1 controls actin dynamics via complex formation with RAC1/CDC42 and PAK1. PLoS Genet 2018; 14:e1007370 PMID: 29734338
- Johnston JJ, van der Smagt JJ, Rosenfeld JA, Pagnamenta AT, Alswaid A, Baker EH, Blair E, Borck G, Brinkmann J, Craigen W, Dung VC, Emrick L, Everman DB, van Gassen KL, Gulsuner S, Harr MH, Jain M, Kuechler A, Leppig KA, McDonald-McGinn DM, Can NTB, Peleg A, Roeder ER, Rogers RC, Sagi-Dain L, Sapp JC, Schäffer AA, Schanze D, Stewart H, Taylor JC, Verbeek NE, Walkiewicz MA, Zackai EH, Zweier C; Members of the Undiagnosed Diseases Network, Zenker M, Lee B, Biesecker LG. Autosomal recessive Noonan syndrome associated with biallelic LZTR1 variants. Genet Med 2018; 20:1175-1185. PMID: 29469822
- Gelb BD, Cavé H, Dillon MW, Gripp KW, Lee JA, Mason-Suares H, Rauen KA, Williams B, Zenker M, Vincent LM. ClinGen's RASopathy Expert Panel consensus methods for variant interpretation. Genet Med 2018; 20:1334-1345. PMID: 29493581
- Altmüller F, Lissewski C, Bertola D, Flex E, Stark Z, Spranger S, Baynam G, Buscarilli de Moraes M, Dyack S, Gillis J, Yntema HG, Pantaleoni F, van Loon RLE, MacKay S, Mina K, Schanze I, Tan TY, Walsh M, White SM, Niewisch MR, García-Miñaúr S, Plaza D, Ahmadian MR, Cavé H, Tartaglia M, Zenker M. Genotype and phenotype spectrum of NRAS germline mutations. Eur J Hum Genet 2017; 25:823-831. PMID: 28594414
- Altmüller F, Pothula S, Annamneedi A, Nakhaei-Rad S, Montenegro-Venegas C, Pina-Fernández E, Marini C, Santos M, Schanze D, Montag D, Ahmadian MR, Stork O, Zenker M, Fejtova A. Aberrant neuronal activity-induced signaling and gene expression in a mouse model of RASopathy. PLoS Genet 2017; 13:e1006684. PMID: 28346493
- Kouz K, Lissewski C, Spranger S, Mitter D, Riess A, Lopez-Gonzalez V, Lüttgen S, Aydin H, von Deimling F, Evers C, Hahn A, Hempel M, Issa U, Kahlert AK, Lieb A, Villavicencio-Lorini P, Ballesta-Martinez MJ, Nampoothiri S, Ovens-Raeder A, Puchmajerová A, Satanovskij R, Seidel H, Unkelbach S, Zabel B, Kutsche K, Zenker M. Genotype and phenotype in patients with Noonan syndrome and a RIT1 mutation. Genet Med 2016; 18:1226-1234. PMID: 27101134
- Boppudi S, Bögershausen N, Hove HB, Percin EF, Aslan D, Dvorsky R, Kayhan G, Li Y, Cursiefen C, Tantcheva-Poor I, Toft PB, Bartsch O, Lissewski C, Wieland I, Jakubiczka S, Wollnik B, Ahmadian MR, Heindl LM, Zenker M. Specific mosaic KRAS mutations affecting codon 146 cause oculoectodermal syndrome and encephalocraniocutaneous lipomatosis. Clin Genet 2016; 90:334-42. PMID: 26970110
- Kratz CP, Franke L, Peters H, Kohlschmidt N, Kazmierczak B, Finckh U, Bier A, Eichhorn B, Blank C, Kraus C, Kohlhase J, Pauli S, Wildhardt G, Kutsche K, Auber B, Christmann A, Bachmann N, Mitter D, Cremer FW, Mayer K, Daumer-Haas C, Nevinny-Stickel-Hinzpeter C, Oeffner F, Schlüter G, Gencik M, Überlacker B, Lissewski C, Schanze I, Greene MH, Spix C, Zenker M. Cancer spectrum and frequency among children with Noonan, Costello, and cardio-facio-cutaneous syndromes. Br J Cancer. 2015 Apr 14;112(8):1392-7. doi: 10.1038/bjc.2015.75. Epub 2015 Mar 5. PMID: 25742478
- Zenker M. Clinical manifestations of mutations in RAS and related intracellular signal transduction factors. Curr Opin Pediatr. 2011 Aug;23(4):443-51. doi: 10.1097/MOP.0b013e32834881dd. Review. PMID: 21750428
TP1 project description - previous funding period FP1








