TP6 - FP1
Analysis of the pathophysiologic link between constitutional RAS pathway activation and epidermal findings in Costello syndrome
Germline missense mutations in the HRAS gene cause Costello syndrome, a rare developmental disorder characterized by a typical facial gestalt, postnatal growth deficiency, intellectual disability, and predisposition to malignancies as well as skeletal, cardiac and dermatological abnormalities. The molecular pathophysiology caused by HRAS mutations has been analyzed in various tissues and cell types; however, up to date the molecular basis for cutaneous manifestations in Costello syndrome is largely unknown. Here we use two novel model systems to address this question, primary skin fibroblasts from patients with Costello syndrome and permanent
human keratinocyte cells carrying an HRAS mutation induced by the CRISPR/Cas9 technology. These cell systems will be used to characterize epidermis-relevant pathophysiological changes and study skin-specific signaling pathways. Thus, we will gain deeper insight into the biology of epidermal homeostasis and its deregulation in Costello syndrome. Deciphering the pathomechanism in all affected tissues is critical to comprehensively understand the molecular etiology of the disorder; this will
lead to scientifically substantiated concepts for the design of pharmacological strategies, which should aid in an improved management of Costello syndrome and related conditions in the near future.
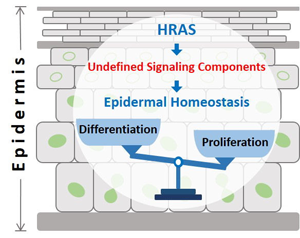 RAS in epidermal homeostasis. HRAS plays a fundamental role in epidermal homeostasis. Inactive HRAS is associated with cell differentiation, whereas activation of HRAS triggers proliferation. Thus, spatially restricted HRAS signaling may divide the epidermis into an undifferentiated proliferative and a differentiating post-mitotic compartment. Yet, the molecular mechanisms and downstream targets of RAS that control epidermal development and homeostasis remain largely unknown.
RAS in epidermal homeostasis. HRAS plays a fundamental role in epidermal homeostasis. Inactive HRAS is associated with cell differentiation, whereas activation of HRAS triggers proliferation. Thus, spatially restricted HRAS signaling may divide the epidermis into an undifferentiated proliferative and a differentiating post-mitotic compartment. Yet, the molecular mechanisms and downstream targets of RAS that control epidermal development and homeostasis remain largely unknown.
Major goals:
Based on our hypothesis that disturbed epidermal homeostasis (proliferation and differentiation) causes the skin phenotype in Costello syndrome, the primary goal of this project is to describe and understand the molecular mechanisms downstream of HRAS that control epidermal development/homeostasis. Therefore, we will …
- … characterize the epidermal pathological changes in Costello syndrome by using gene-edited permanent human keratinocyte (HaCaT) cell lines and primary skin fibroblasts of patients with Costello syndrome as model systems.
- … study signaling pathways associated with epidermal homeostasis - with a special focus on integrin regulation - by using various molecular/cell biological techniques.
Publications:
- Brand K, Kentsch H, Glashoff C, Rosenberger G. RASopathy-associated CBL germline mutations cause aberrant ubiquitylation and trafficking of EGFR. Hum Mutat. 2014 Nov;35(11):1372-81. PMID: 25178484
- Lorenz S, Lissewski C, Simsek-Kiper PO, Alanay Y, Boduroglu K, Zenker M, Rosenberger G. Functional analysis of a duplication (p.E63_D69dup) in the switch II region of HRAS: new aspects of the molecular pathogenesis underlying Costello syndrome. Hum Mol Genet. 2013 Apr 15;22(8):1643-53. PMID: 23335589
- Rosenberger G, Meien S, Kutsche K. Oncogenic HRAS mutations cause prolonged PI3K signaling in response to epidermal growth factor in fibroblasts of patients with Costello syndrome. Hum Mutat. 2009 Mar;30(3):352-62. PMID: 19035362








